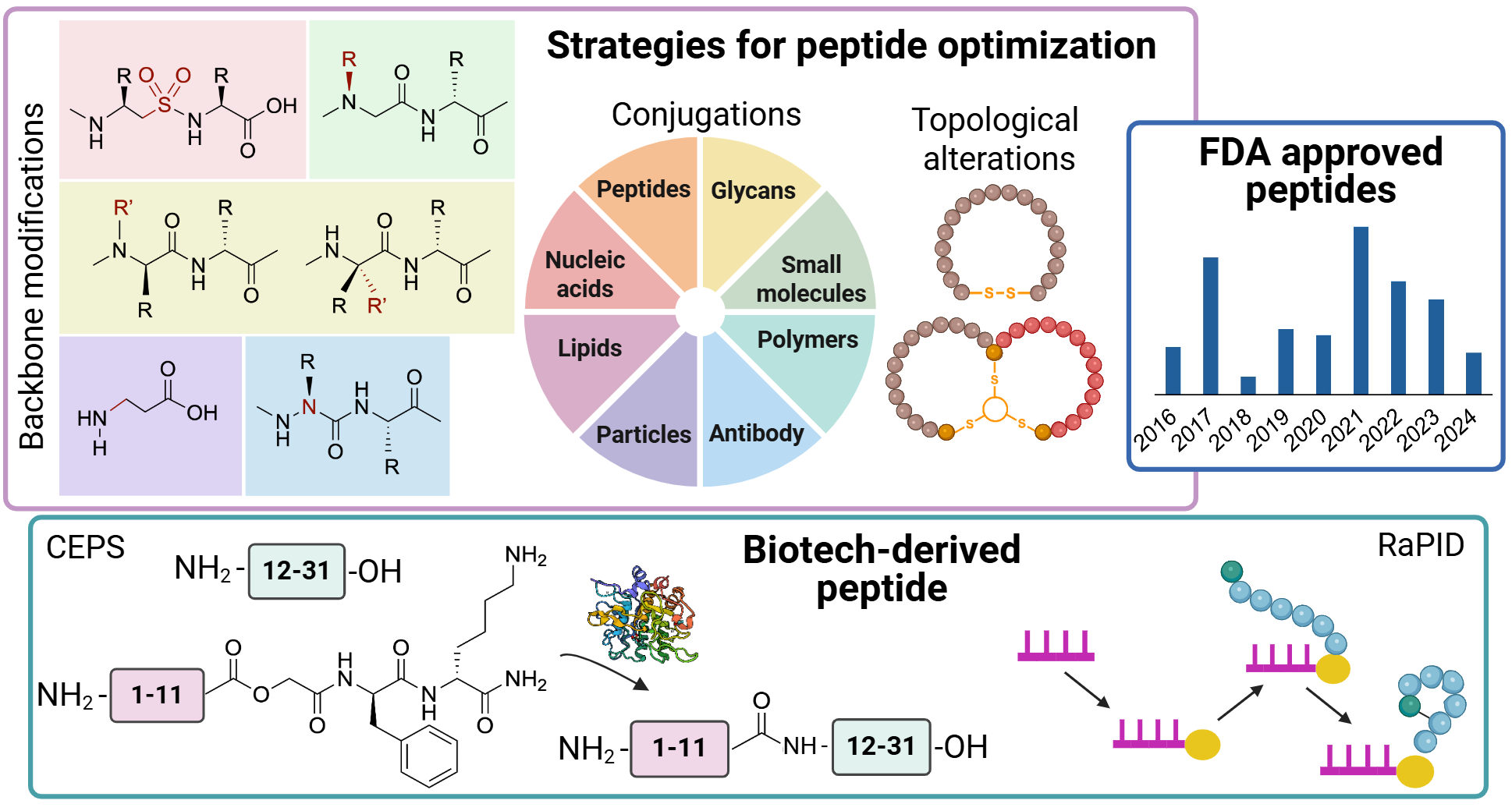
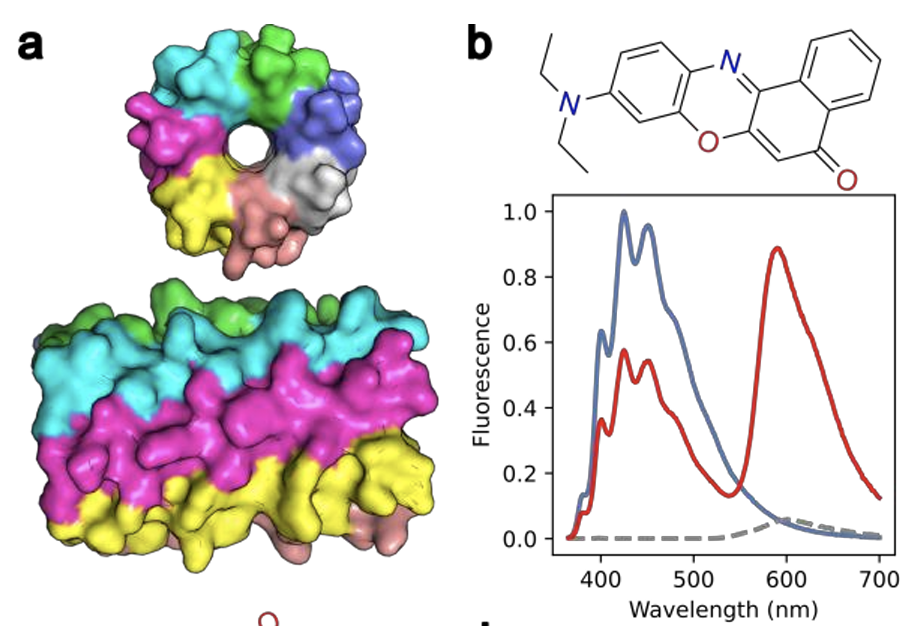
Cutaneous melanoma, responsible for 80% of skin cancer mortality, presents urgent diagnostic challenges due to insufficient early detection methods. Current clinical methods rely on invasive biopsies, while noninvasive approaches primarily serve as adjunctive decision-support tools rather than definitive diagnostics. Here, a peptide-based fluorescent biosensing system was developed for the sensitive and rapid detection of S100B, a key prognostic biomarker for melanoma. Our system employs a fluorescently labeled peptide beacon designed for Förster resonance energy transfer (FRET)-based detection, achieving a subnanomolar detection limit (∼0.045 nM) and great selectivity in human serum samples. Peptide synthesis was performed using optimized solid-phase protocols, enabling precise sequence assembly, while the peptide sensor offers efficient detection, lower costs, and high specificity through tailored peptide–protein interactions. The biosensing probe employs complementary peptide nucleic acid (PNA) interactions to achieve proximity-induced fluorescence quenching in the absence of S100B, which reverses via structural rearrangement upon specific S100B binding for accurate quantification. Computational and experimental optimization of the synthetic process has enhanced binding efficiency, sensitivity, and response time–crucial parameters for melanoma-specific detection. By integrating advanced molecular design with optical biosensing, this mechanism aims to enhance the accuracy and accessibility of melanoma diagnostics, ultimately addressing healthcare disparities and improving patient outcomes.
The work is published in Bioconjugate Chemistry and can be found here: